Regulatory and Approval Information
Natamycin is a food preservative that is approved and used in more than 150 countries around the world. The World Health Organization (WHO), European Food Safety Authority (EFSA) and Food and Drug Administration (FDA) have evaluated Natamycin thoroughly and all list it as safe for human consumption.
In the map below some examples of approval of Natamycin in major countries and at global level can be found. In the publication center you will find the relevant regulatory documents and safety overviews.
Latest notification: FDA approves Natamycin in non-alcoholic beverages. COFEPRIS (Mexico) approves Natamycin in yogurt and non-alcoholic beverages.
Labeling of Natamycin
In the European regulation on food additives, the natural mold inhibitor Natamycin is described as a preservative (E 235) for the surface treatment of hard, semi-hard and semi-soft cheeses, and of dried, cured sausages. Mandatory labeling is necessary where it is not used on the rind, but directly on the cheese surface. In some countries outside Europe E numbers are not used and Natamycin can be labeled as Natamycin (a natural mold inhibitor) or as Pimaricin (a natural preservative).
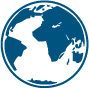
Based on different applications, most countries in the world have approved Natamycin for preserving food products: from Canada to China and South Africa.

